
In this equation, is the intrinsic viscosity that's the viscosity attributed to the solute, rather than the viscosity of the solvent itself. In this case, the relationship is described by the Mark-Houwink equation: That means that one quantity depends on the other raised to some exponent. Often in nature one physical property that depends on another will follow a "power law". That approach will often give a straight line when two things are related. One common thing to do in this situation is to take the log of the values. That can make it more difficult to use a graph like this to predict molecular weight from a viscosity measurement. So, the viscosity increases with the molecular weight, but not necessarily in a linear way. The larger the polymer, the more drag and also the more intermolecular attraction, and so the higher the viscosity. In fact, viscosity measurements of polymer solutions are another way to determine the size of the polymer - leading to the chain length and the molecular weight. Increased drag is also a factor even in solution, these very large molecules encounter more resistance as they move past solvent molecules compared to the resistance smaller molecules would experience. The extended contact between their long molecular chains leads to enhanced intermolecular attractions that contribute to a resistance to flow. Those longer hydrocarbon chains in the motor oil cling to each other much more strongly than the shorter ones in the gasoline, so gasoline flows much more easily than motor oil.įor similar reasons, oligomers and polymers, if they are liquids, also display high viscosity. The amount of intermolecular interactions is also crucial in determining how tightly two molecules hold onto each other, and this factor is especially important in very weak London dispersion forces, where a small advantage goes a long way.
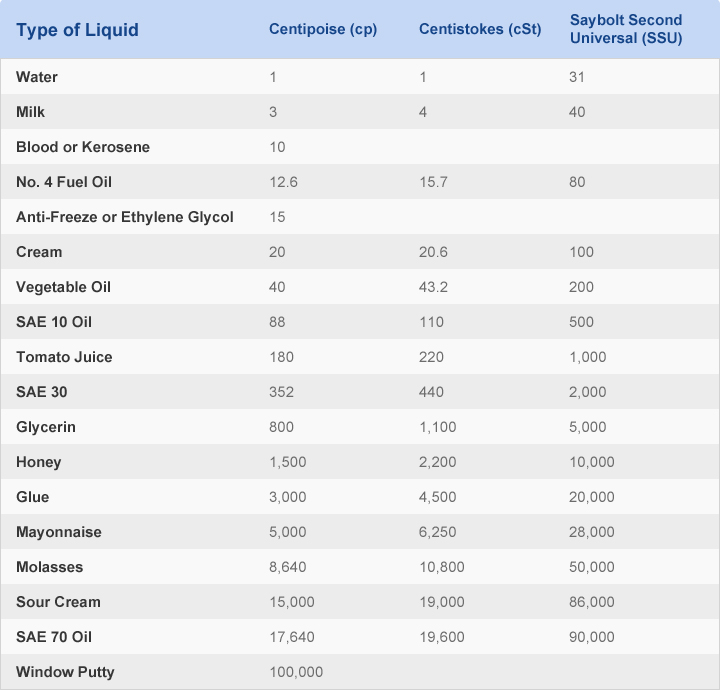
The molecules are bigger, and so they experience more drag as they move through the liquid. The major difference between motor oil and gasoline is that the motor oil contains much longer hydrocarbon chains. The intermolecular attractions between the molecules are much weaker than those between sugar molecules they're just London dispersion forces. Motor oil and gasoline are composed of the same class of compounds, hydrocarbons, composed of carbon chains covered in hydrogen atoms. The motor oil example works in a similar way. As these molecules move past each other in the very concentrated solution, they cling to each other, slowing down the flow of the liquid. Sugars are covered in OH or hydroxyl groups that are capable of strong hydrogen bonds. The strong intermolecular attractions between the individual sugar molecules are also a major factor. Why is honey so viscous? Partly it's just that the sugar molecules in the water are much larger than the water molecules, so they experience a lot more drag as they move through the solution compared to just plain water. Nevertheless, this high viscosity is something honey has in common with polymer solutions, as well as with some oligomers, which are short-chain polymers that can be liquids instead of solids. The sugars aren't polymers, either they are simple monosaccharides such as glucose and fructose.
ZING POINT OF HIGH VISCOSITY LIQUIDS PLUS
It contains a little bit of water and a whole lot of sugars, plus other small molecules produced by the plants from which the bees gathered the nectar to make the honey. The honey doesn't flow very easily, especially compared to something like water. Viscosity is often described in very general terms as "resistance to flow". It resists the movement of the spoon when we stir it. When we say that, we mean that water is much easier to stir or to pour than honey. For example, we say that honey is more viscous than water. Viscosity is a term we use to describe the "thickness" of different liquids. Let's start with a property that you are probably familiar with. What makes them unique? Why do they have properties that aren't easily replicated by other materials? Polymers have come to occupy a very important niche in the materials we use every day.


 0 kommentar(er)
0 kommentar(er)
